Collecting ACR Method of Detection (MOD) data is a hot topic among breast cancer experts. In contrast to other countries, the U.S. has no systematic method for gathering MOD data, nor a standardized framework for reporting it. Fortunately, the American College of Radiology (ACR) is aiming to change that, and at Magview, we’ve quickly adapted our software to make the collection of this data a simple and automated process.
Why should you track ACR Method of Detection?
The ACR defines the initial MOD as the “first imaging test or sign or symptom that triggered the subsequent workup and diagnosis of breast cancer.”
“When national service-screening programs and registries were built in the 1980s and 1990s, the choices for initial MOD were limited,” the ACR says. “Screen-film mammography was the only image-based screening test. Today, initial MOD can include multiple other image-based screening modalities.”
The organization lists several important reasons to track MOD, noting that:
- “Many other nations track the performance and results of screening mammography for every patient.
- USA cancer registries do not include MOD data or a direct registry link from the MOD to specific patients and their outcomes.
- Mortality from breast cancer continues to decrease in the USA but the debate over when to start and how often to screen patients rages on in the USA.
- Without tracking MOD, we cannot fully understand the fundamental impact of screening, or the lack thereof, on treatment and outcomes in diverse racial and socioeconomic communities in the USA.”
According to the ACR, if MOD can be “assigned and collected accurately and without bias for each patient,” that would provide “new primary data, rather than models based on historic data that may no longer accurately represent the diversity of our screening-eligible population or advances in screening technologies.”
“Concrete, patient-specific data could bring the ACR, USPSTF and ACS to consensus recommendations for screening,” the ACR says. “We could employ the MOD-inclusive data to answer numerous national population-based questions about how screening relates to efficacy, equity, treatment, and breast cancer.”
How to track ACR Method of Detection?
According to the ACR, tracking MOD starts with asking, “What was the first thing to trigger the work-up that ultimately led to the recommendation for biopsy?”
The MOD is then assigned based on “prior imaging and clinical criteria.”
There are three basic categories for initial MOD:
- S – image-based Screening detection in asymptomatic patients — which includes diagnostic or screening exams for asymptomatic patients in follow-up after completing treatment
- P – Patient detected by self-exam or symptom, or Provider detected with clinical exam
- N – None of the above OR Not S and Not P (distant metastasis, CT, labs, non-breast biopsy, etc.)
Each has specific sub-categories, which are captured in ACR’s One Page Quick Reference.
How MagView supports the collection of ACR Method of Detection data
MagView has you covered when it comes to collecting MOD data, providing a simple and automated approach.
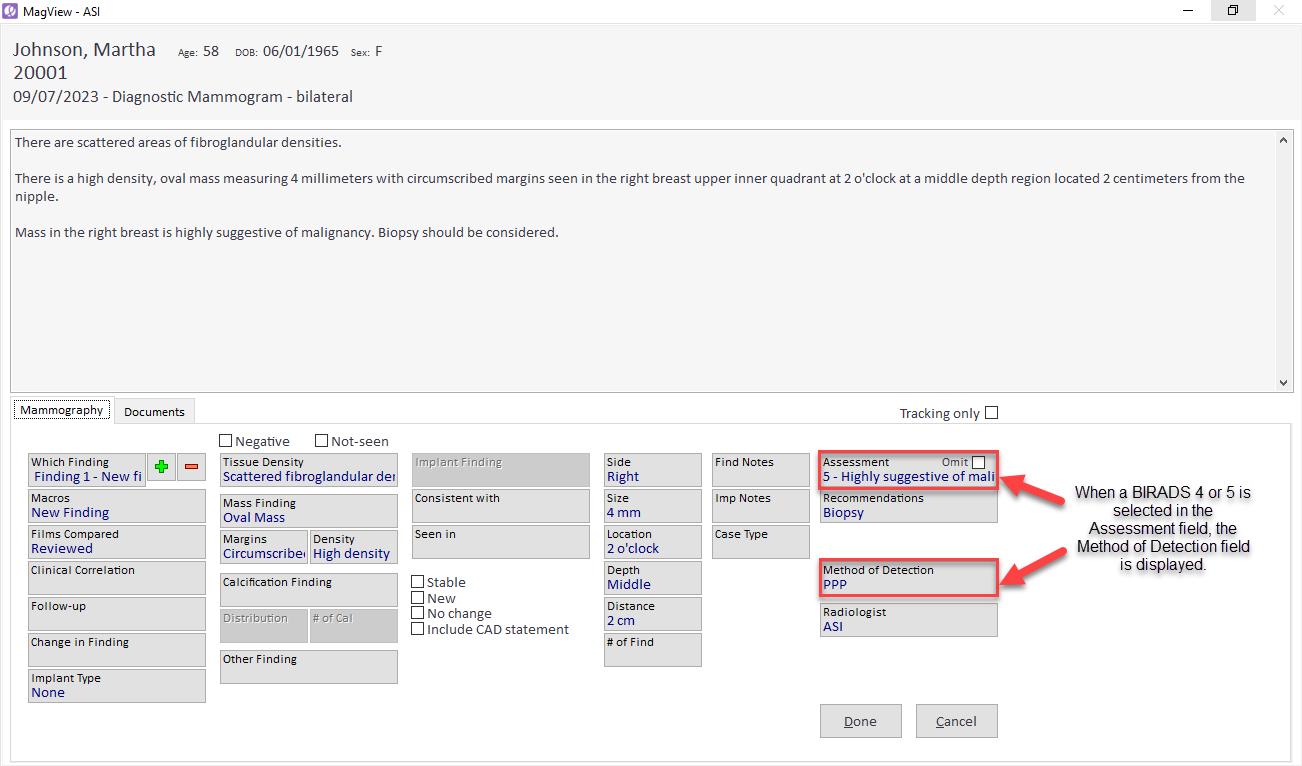
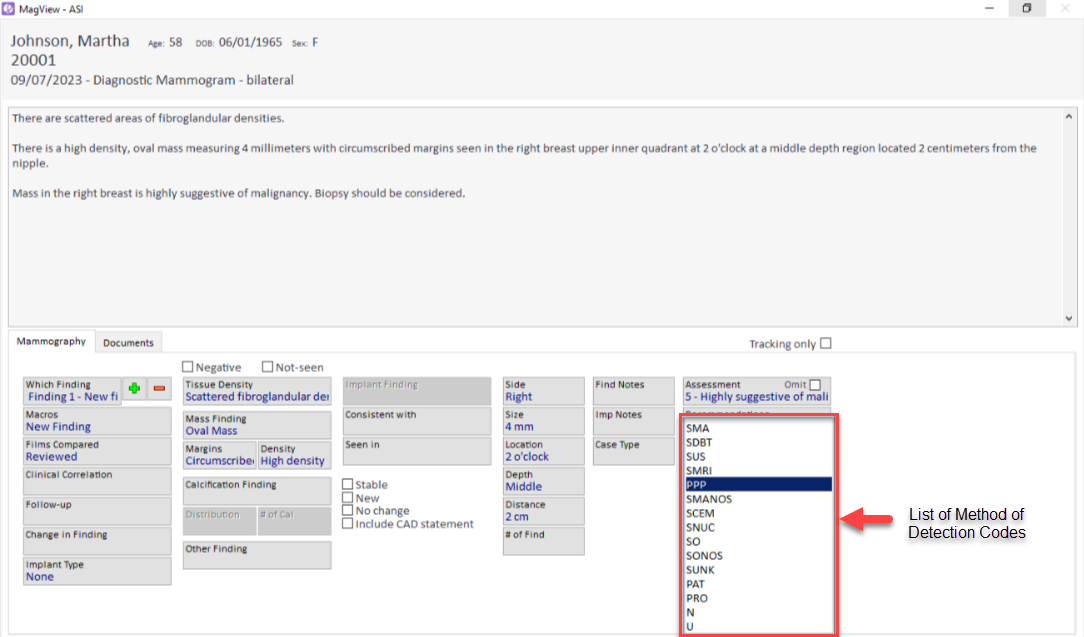
When a radiologist logs findings classified as BI-RADS 4 or 5, the system automatically presents the default MOD. This is enabled by MagView’s advanced algorithm that parses existing information within the system and then maps it to the defined MOD categories per ACR guidelines.
Should the radiologist wish to modify the MOD, MagView provides the flexibility to do so.
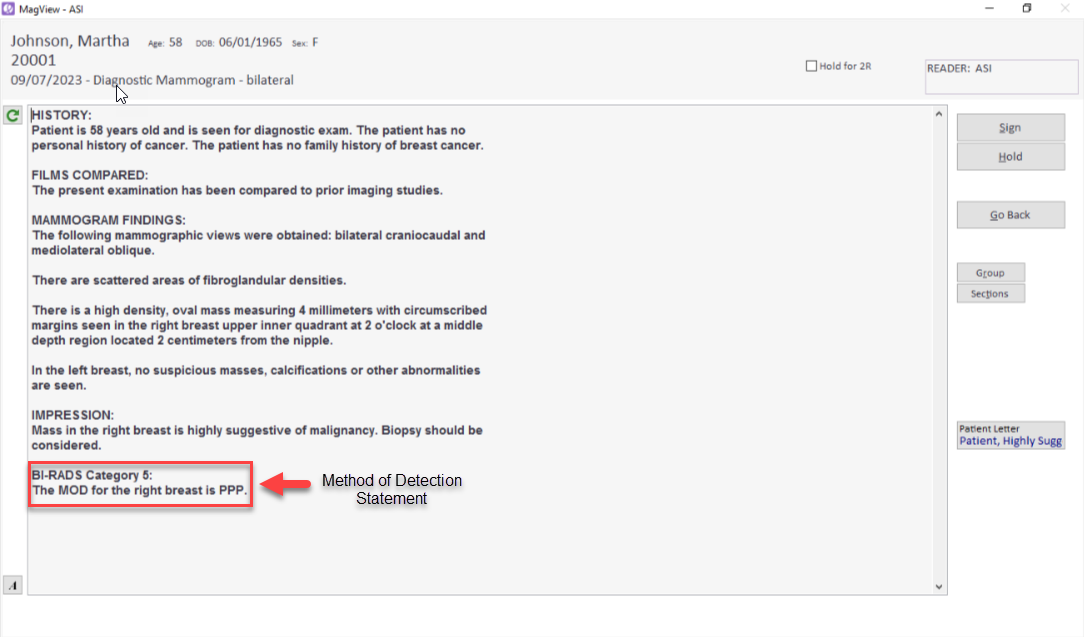
MOD details are then integrated seamlessly into the final report.
MagView’s robust capacity to automate MOD data collection and assignment in alignment with National Mammography Database submission (NMD v. 3.4) requirements provides a distinct advantage for breast centers.
Even better, this advanced functionality can be effortlessly implemented through configuration adjustments within your existing setup, eliminating the need for additional updates.
Navigating new 2024 regulations for breast centers— including ACR Method of Detection
As the MQSA regulations approach in 2024, alongside new NAPBC risk assessment criteria and the ACR Method of Detection measure, staying informed is crucial to compliance.
Our upcoming webinar — Navigating New Regulations in 2024: A Guide to Compliance for MQSA, NAPBC, and MOD — provides an opportunity to gain expert insight into these pivotal regulatory changes and understand the practical steps necessary to ensure compliance.
We’d love to have you join us, so please register today.
















![monitoring breast density shutterstock_1299510538-[Converted]](https://magview.com/wp-content/uploads/2023/05/shutterstock_1299510538-Converted.jpg)