Contrast enhanced mammography (CEM) is growing in popularity to aid in breast cancer screening and diagnosis. CEM offers a number of benefits, which is why breast centers are increasingly adding this method of detection to their offerings. That’s good news for the women who can benefit — as well as the breast centers using CEM to expand their reach.
And more good news? MagView will soon be adding contrast enhanced mammography reporting to our mammography tracking and reporting software, making it easier for breast centers to integrate it into their daily workflows.
What is contrast enhanced mammography?
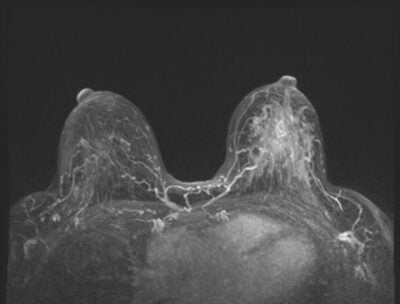
Approved by the U.S. Food and Drug Administration (FDA) in 2011 as an adjuvant to mammography, CEM involves the injection of contrast media during mammography to help diagnose breast cancer. Since angiogenesis plays a role in breast cancer development, enhanced neovascularity on imaging can help identify and evaluate areas of concern. According to DenseBreast-info, the FDA’s approval is for diagnostic breast imaging, and its use for screening is considered “off-label” and is an “area of active investigation.”
Over the years, CEM techniques have improved and include the current dual-energy technique, which is succinctly described in a November 2022 European Journal of Radiology article, “Contrast-Enhanced Mammography in Breast Cancer Screening.”
“CEM employs a dual energy technique that capitalizes on the difference in X-ray attenuation between breast tissue and iodine when low and high energy spectra are used,” the authors write. “CEM examinations are comprised of paired low-energy and high-energy images obtained from utilizing X-ray energies below and above iodine’s k-edge (33 keV), respectively. From these paired exposures acquired in the same compression, a recombined image is generated using a post-processing algorithm that isolates the iodine concentration only.”
The article also notes that in 2020, the FDA approved the first commercial system to enable CEM-guided biopsy.
The growing popularity of contrast enhanced mammography
A growing body of research indicates that CEM may provide results comparable to contrast-enhanced breast magnetic resonance imagery (MRI), a dynamic contributing to its surge in use.
In fact, CEM is becoming so popular that, in 2022, the American College of Radiology (ACR) published a supplement to ACR BI-RADS® Mammography 2013 that specifically addresses this modality.
“Contrast enhanced mammography (CEM) was first approved by the Food and Drug Administration (FDA) in 2011 and its use is increasing,” ACR says. “CEM has been shown to be more sensitive than mammography or ultrasound for the detection of malignancy. Given that utilization is increasing, it is important that a lexicon be available to allow for consistency in reporting and also to allow for validation of standardized terms through studies looking at the performance of CEM in a variety of clinical settings. The next edition of BI-RADS® is under development but rather than waiting to issue a section on CEM until the release of the new edition, this supplement is available to facilitate the interpretation and reporting of CEM studies.”
Benefits of contrast enhanced mammography
Many of the benefits associated with CEM relate to its potential as an alternative to breast MRI, including:
- Reduced cost — which may improve compliance for women facing high out-of-pocket costs for recommended supplemental testing
- Increased patient convenience — since patients can receive testing at the breast center, rather travel to another facility
- Improved patient comfort — which is especially true for patients who are claustrophobic or sensitive to other MRI-related factors, such as high noise levels
- Expanded screening access — for high-risk women who may benefit from supplemental screening, but cannot have an MRI (see the ACR’s recommendations about CEM in its updated breast cancer screening guidelines)
- Enhanced business growth — since CEM-capable breast centers can offer additional screening in house, rather than sending patients to another setting
Additionally, like breast MRI, CEM is considered a reliable screening and diagnostic tool for patients with dense breasts who may have lesions not detectable with mammography alone.
Contrast enhanced mammography reporting: Coming soon to MagView
In light of the growing popularity of CEM, breast centers around the country may opt to integrate it into their offerings. To do so effectively, it will be important to streamline workflow when it comes to radiologist reporting — which is where MagView comes in.
MagView is well-known as the leading provider of solutions for breast center information management. As a result, we understand the critical importance of effective data integration to optimize the mammography workflow — which is why CEM reporting will soon be part of the mix.
In the near future, we’ll be offering the same great benefits of structured reporting for CEM that our customers are familiar with for mammograms, ultrasounds — and in some cases — MRI. Our structured reporting ensures reports that are concise, consistent, and compliant with regulatory requirements.
Stay tuned for our official announcement about our new CEM offering, which will include further details about related reporting capabilities.












![monitoring breast density shutterstock_1299510538-[Converted]](https://magview.com/wp-content/uploads/2023/05/shutterstock_1299510538-Converted.jpg)