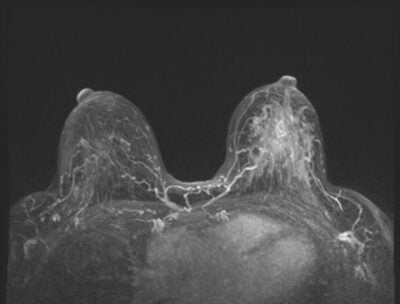
Assessing breast density and informing patients of their findings are critical components of quality breast care. This process is so important that the majority of states in the U.S. have dense breast laws on the books to help ensure patients receive the information they need. Additionally, the FDA recently announced changes to the Mammography Quality Standards Act (MQSA) making breast density notification a federal requirement, effective September 10, 2024.
However, complying with evolving legislation can be easier said than done—unless cutting-edge mammography reporting and tracking software like MagView is part of the workflow. In the following, we’ll show you how MagView handles breast density notification and how we can help breast centers comply with dense breast notification legislation.
What Are Dense Breast Laws?
State-level breast density notification (BDN) laws are the result of a grass-roots effort started by Nancy M. Cappello, Ph.D., who was diagnosed with stage 3C breast cancer in 2004 after having a “normal” mammogram six weeks prior. When she questioned why the cancer wasn’t discovered on a mammogram, she learned that she had dense breasts and that the increased breast density masked the lesion. Additionally, her increased density also placed her at higher risk for developing breast cancer.
Breast Density Awareness
During her quest to learn more, Dr. Capello discovered that although the role of breast density in breast cancer was well-established in related research, most women weren’t being provided with information about their breast density so they could advocate for themselves. To increase breast density awareness, Dr. Capello founded Are You Dense, Inc, and Are You Dense Advocacy, Inc. with her husband Joseph J. Capello.
Legal Notification Methods
After years of effort, the first BDN law was enacted in Connecticut in 2009. According to Dense Breast-Info, breast density notification of some type is now required in 38 states and the District of Columbia (as of January 29, 2023). However, the organization also notes that some state laws may only require that a general notification about breast density be sent instead of notifying a woman about her own breast density status. Additionally, “though some state laws are more similar than others, there is no standard from state-to-state on what patients are told or how patients will be informed.”
The good news is that legislative changes recently announced by the FDA aim to change all that.
New Federal Legislation
The U.S. Food and Drug Administration (FDA) made important recommendations about breast density in 2019 when it proposed that breast density notifications be included in the requirements of the MQSA. On March 9, 2023, the agency announced that an update to the regulations had been finalized.
“Today, the U.S. Food and Drug Administration published updates to the mammography regulations to, among other things, require mammography facilities to notify patients about the density of their breasts, strengthen the FDA’s oversight and enforcement of facilities and help interpreting physicians better categorize and assess mammograms,” according to the press release.
Noting that the final rule “amends regulations issued under the Mammography Quality Standards Act (MQSA) of 1992,” the FDA referred to the new breast density notification requirements as one of the “key” updates.
“Approximately half of women over the age of 40 in the U.S. have dense breast tissue, a description of its appearance on a mammogram,” the FDA said. “Dense breast tissue can make cancers more difficult to detect on a mammogram. Additionally, dense breasts have been identified as a risk factor for developing breast cancer. The amendments finalized today provide specific language explaining how breast density can influence the accuracy of mammography. They recommend patients with dense breasts talk to their health care provider about breast density, risks for breast cancer and their individual situation.”
The final rule provides additional information about the changes, including the following from the Executive Summary:
- “This final rule requires that the summary of the mammography report written in lay terms (‘lay summary’) that is provided to patients identifies whether the patient has dense or non-dense breast tissue and includes a prescribed paragraph on the significance of breast density.”
- “The rule also establishes four categories for reporting breast tissue density in the mammography report that is provided to the patient’s referring healthcare provider.”
In a summary of the major provisions of the final rule, the FDA notes that although the two categories of density in the patient lay summary are being retained, the wording is being changed from “high density” and “low density” to “dense” and “not dense,” which the FDA says better aligns with clinical practice and provides more clarity for patients.
Additionally, the written lay summary of results provided to the patient must contain one of the following statements:
- Non-dense breast notification: “Breast tissue can be either dense or not dense. Dense tissue makes it harder to find breast cancer on a mammogram and also raises the risk of developing breast cancer. Your breast tissue is not dense. Talk to your healthcare provider about breast density, risks for breast cancer, and your individual situation.”
- Dense breast notification: “Breast tissue can be either dense or not dense. Dense tissue makes it harder to find breast cancer on a mammogram and also raises the risk of developing breast cancer. Your breast tissue is dense. In some people with dense tissue, other imaging tests in addition to a mammogram may help find cancers. Talk to your healthcare provider about breast density, risks for breast cancer, and your individual situation.”
Also, the written report of mammography results provided to the healthcare provider must include information “concerning an overall assessment of breast density,” which must be classified in one of the following categories:
- “The breasts are almost entirely fatty.”
- “There are scattered areas of fibroglandular density.”
- “The breasts are heterogeneously dense, which may obscure small masses.”
- “The breasts are extremely dense, which lowers the sensitivity of mammography.”
In addition to providing other benefits, the FDA says the amendments “help bring the MQSA into the 21st century, including modernizing the regulations by incorporating current science and mammography best practices to improve breast cancer detection and helping empower patients with more information when they are considering important decisions regarding their breast health care.”
Along with other changes to the MQSA regulations, the new breast density notification requirements must be implemented within 18 months of publication of the final rule.
How MagView Collects Breast Density
MagView has helped breast centers communicate to patients about breast density since the first law was enacted in 2009. We’ll continue to support our customers as they make changes or additions to their breast density assessment and patient communication workflow.
MagView can collect breast density in several ways without any extra steps in the workflow.
- The breast density category can be automatically extracted from the report dictated by the radiologist.
- If MagView structured reporting is used, the radiologist will choose the breast density category from a drop-down menu or the tissue density category can carry forward from the previous visit.
- If a site is using automated breast density assessment software, the category can be sent to MagView via an integration.
MagView ensures that breast density classification is always collected, no matter which reporting method is used.
How MagView Handles Breast Density Notification in Patient Letters
Based on the patient’s breast density classification, a breast density statement will automatically be added to the patient’s lay letter. There are no extra steps to include breast density verbiage in the patient letters, saving staff time. Patient letters can be sent through the mail, or some facilities opt to send patient letters electronically via the Patient Results Portal for quicker delivery and a more positive patient experience.
The Importance of Density Assessment Software Integration
Density assessment software is a popular tool used by many breast centers to enable objectivity when assessing a patient’s breast density. However, if MagView is in use and the two aren’t integrated, a reporting gap may occur. That’s why an important way breast centers can improve breast density notification is by integrating their breast density assessment software with MagView. Bridging that gap will automatically populate:
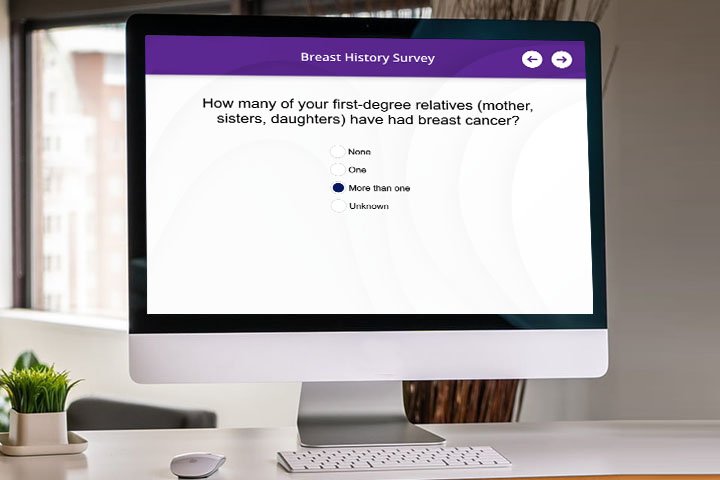
- Tyrer-Cuzick v8—IBIS v8 includes the patient’s breast density as part of the risk assessment calculation. This integration will produce a real-time Tyrer-Cuzick score (Risk Assessment Module with Tyrer-Cuzick required).
- BI-RADS Report—Breast composition is required in the BI-RADS report. This integration means one less click for the radiologist (If using MagView Structured Reporting, the radiologist may overwrite the software partner’s density assessment with their own assessment).
- Patient Letter—If the category reported by the software or the radiologist is “dense,” a dense breast statement will automatically be inserted into the patient’s lay letter.
At MagView, we provide the support you need to ensure excellence in patient care and adherence to compliance within an evolving regulatory landscape.
Contact us today to learn more.












![monitoring breast density shutterstock_1299510538-[Converted]](https://magview.com/wp-content/uploads/2023/05/shutterstock_1299510538-Converted.jpg)