The days of ambiguous breast density notification for mammography reporting are finally coming to an end. On March 10, 2023 The Food and Drug Administration (FDA) issued a final rule to update mammography regulations under the Federal Food, Drug, and Cosmetic (FDC & C Act) and Mammography Quality Standards Act of 1992(MQSA).
This update aims to modernize regulations by incorporating best practices and current scientific knowledge to improve mammography services and strengthen healthcare information communication for more informed decision-making by providers and patients.
Breast healthcare facilities can utlize modern technology, such as Magview’s Mammography Software Systems & Solutions, to deliver meaningful, actionable data to patients.
The Update
By September 10, 2024, breast health institutions will be required to provide standard breast density notification in mammography reports. In addition to requiring notification in the patient summary of mammography reports regarding whether a patient has dense breasts, the rule also mandates standard language indicating the limitations of mammography for women with dense breasts and promoting additional imaging.
Standard breast density notification language
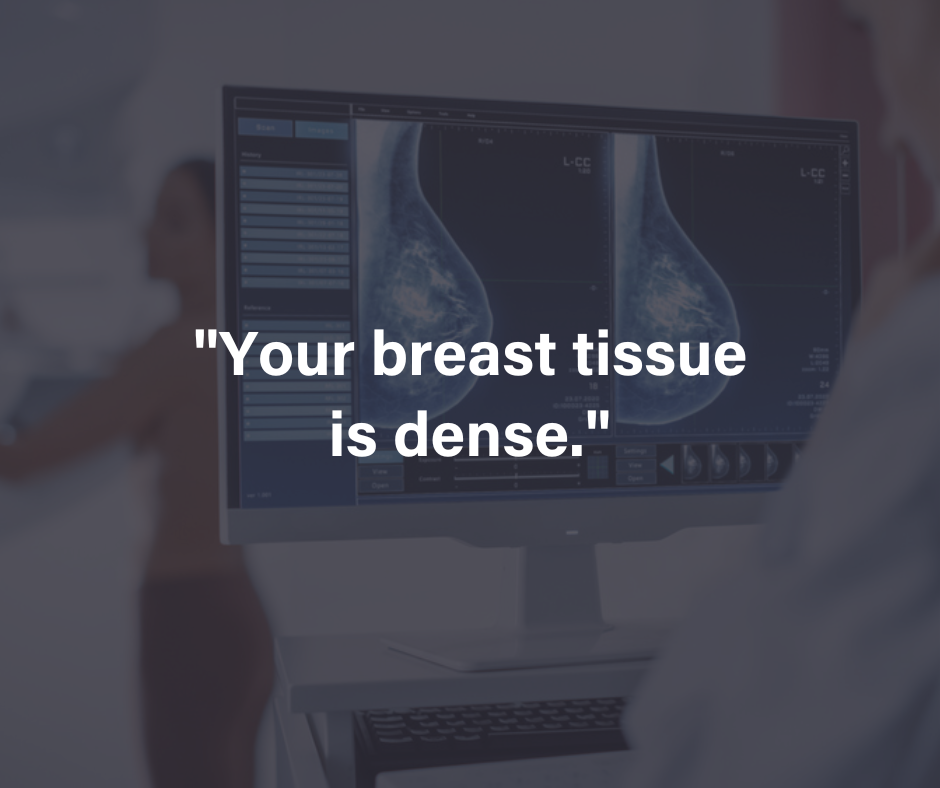
The FDA requires that breast density notifications either indicate “dense” or “not dense.” Dense tissues can generally hide cancer, and having dense breasts can increases one’s risk of breast cancer. For this reason, women with dense breasts are encouraged to undergo supplemental imaging. Women in the US will receive a clear notification if their breasts are dense and that having dense breasts increases the risk of breast cancer.
What is Breast Density?
Now that your mammogram will include information about your breast density, it’s essential to understand how relevant breast density is to your breast health.
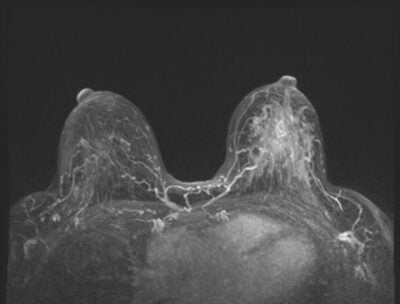
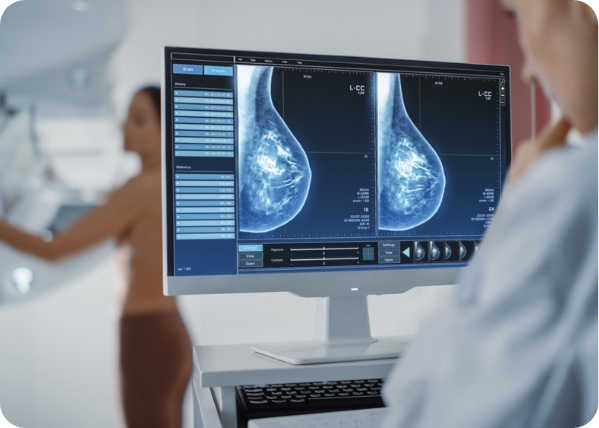
Breast density refers to the proportion of glandular and fibrous tissue in a woman’s breast compared to the amount of fatty tissue, as seen in a mammogram. Generally, the more fatty tissue your breast has, the less dense they are. In a mammogram, fatty tissues appear darker, making it easier to view the entire breast without issues. However, if the breasts have more glandular and fibrous tissues than fatty tissues, the whole breast will appear white – as will a potential tumor – making it difficult to distinguish between the tissues and the tumor in a mammogram. Consequently, a small tumor could be missed, and by the time it’s detected, it may be too large to treat effectively.
The Benefits of Getting Breast Density Notification in the Mammography Report
Breast health experts have applauded the rule by FDA, saying it will improve the overall breast health for women in the United States. The prevalence of dense breasts in approximately 40 percent of women in the United States makes this rule essential in the fight against breast cancer.
Breast density plays a massive role in determining a person’s risk of breast cancer. Dense breasts are among the risk factors for breast cancer, as doctors must incorporate other screening methods to detect breast cancer. The greater the amount of dense tissue, the higher the risk. However, it’s critical to note that having dense breasts doesn’t necessarily mean you have cancer. Similarly, having less dense breasts doesn’t mean you’re cancer-free. It only means that it’s easier to spot it on a mammogram.
Now that you know the importance of breast density and what it means for your breast health, why not start assessing your risk? We have various resources, including the IBIS calculator, to help you calculate your risk. Check it out on our website.
References
- https://www.diagnosticimaging.com/view/fda-issues-final-rule-on-national-breast-density-notification-for-mammography-reports
- https://public-inspection.federalregister.gov/2023-04550.pdf













![monitoring breast density shutterstock_1299510538-[Converted]](https://magview.com/wp-content/uploads/2023/05/shutterstock_1299510538-Converted.jpg)